Screening for Colorectal Cancer
Sections on this page:
- Screening Tests Included in the Models
- Historic and Projected Screening Utilization
- Model Inputs for Screening
- How the Effect of Screening is Modeled
Summary: learn which tests for colorectal cancer we modeled and how
The early detection of colorectal cancer (CRC) is an important cancer control strategy, both because it can relatively quickly reduce mortality, by finding disease early when it can be most easily treated and because, when pre-cancerous lesions (i.e., adenomas) are identified and removed, CRC is prevented.
Several tests are recommended for CRC screening including the fecal occult blood test (FOBT) done at home by the patient, flexible sigmoidoscopy done in a physician’s office, and colonoscopy usually done with sedation in the office of a gastroenterologist.
The overall use of at least one of these tests, while rising, remains relatively low compared to mammography screening for breast cancer and Pap smear testing for cervical cancer. The models examine how different assumptions about improved CRC screening can impact CRC mortality.
The output of these simulations with improved screening can then be compared with other runs where risk factors or chemotherapy were modeled. Using this technique, the models show screening to be the most effective method, among current choices, for reducing CRC mortality over the next 10 or 15 years. You can compare graphs of the model output for various runs in the Interactive Graph section.
Screening Tests Included in the Models
The two simulation models incorporate the effects of commonly-used screening tests to assess the impact on colorectal cancer (CRC).
- Fecal Occult Blood Test – Looks for evidence of occult (hidden) blood in the stool. A positive fecal occult blood test requires follow-up colonoscopy. We model FOBTs performed by the patient at home; FOBTs performed in the physician’s office have been found to be ineffective and are no longer recommended.
- Sigmoidoscopy or Colonoscopy (collectively known as Endoscopy) – Uses a camera on a flexible tube introduced through the anus to examine the colon and rectum for abnormal growths (polyps) called adenomas. Sigmoidoscopy is performed on an alert patient, and reaches at most the first third of the large colon. Any polyps detected are recorded (and may be biopsied) and the patient is generally referred for colonoscopy. Colonoscopy is a more involved procedure, performed on a sedated patient, which permits examination of the entire length of the colon. During colonoscopy, any suspicious polyps can be removed, which may prevent an occurrence of cancer in the future.
Historic and Projected Screening Utilization
The models require input as to the percentage of the population receiving each screening test each year. There are no direct statistics giving that information, but the National Health Interview Survey (NHIS) reports several related statistics:
- percentage of population reporting having had a home FOBT, and how long ago; and
- percentage of population reporting having had a colorectal exam of any sort.
The National Health Interview Survey did not distinguish between home and office FOBTs until the 2000 survey. Starting with the 2003 survey, sampled adults were asked only about home FOBT use. Respondents were first asked whether they had had a sigmoidoscopy or colonoscopy in the 2000 National Health Interview Survey. Prior to that, the question was about proctoscopy, a procedure no longer recommended for CRC screening.
In order to transform this screening data into model inputs, we derived estimates for year by year rates of home FOBT, sigmoidoscopy and colonoscopy.
We used this information to determine the screening utilization for the various projection scenarios.
- Projected Trends rates assume the current rate of change will remain constant.
- The assumption underlying Optimistic but Realistic rates is that a screening level comparable with breast cancer (70% up-to-date with screening) is possible by 2010. Screening could be either FOBT in the past year, sigmoidoscopy in the past 5 years or colonoscopy in the past 10 years.
- The Healthy People 2010 objectives are that 50% of adults 50 years of age or older would have received colorectal cancer screening by 2010. We calculated trend lines to reach that objective, starting at the estimated 2004 level. Note that in some cases the projected trends and optimistic goals for endoscopy are higher than the Healthy People 2010 target of 50%.
For more discussion of current trends in colorectal cancer screening, see the Early Detection section of the Cancer Trends Progress Report.
Model Inputs for Screening
The following table shows screening model inputs in 2010 for each race/sex combination. Items marked with an asterisk (*) vary from the corresponding Healthy People 2010 objective. For instance, the actual HP 2010 objective is specific to sigmoidoscopy. We count both sigmoidoscopy and colonoscopy, since the current recommendation is for either.
| Screening Input | Race/Sex Group (Click for graph of input projected through 2020) |
2000 Level | Level in 2010 | ||
|---|---|---|---|---|---|
| Projected Trends | Optimistic but Realistic | HP 2010 (Click for more about objective) |
|||
* Statement of objective varies from Healthy People 2010 |
|||||
% over age 50 have FOBT within past 2 years |
white male | 23% | 17% | 22% | 33% |
| white female | 25% | 19% | 23% | ||
| black male | 22% | 19% | 22% | ||
| black female | 22% | 20% | 24% | ||
% over age 50 have had a sigmoidoscopy
or colonoscopy (collectively known as endoscopy) at some point in their life * |
white male | 44% | 60% | 63% | 50% (60%)** |
| white female | 38% | 56% | 59% | ||
| black male | 40% | 48% | 50% | ||
| black female | 32% | 52% | 55% | ||
How the Effect of Screening is Modeled
In the absence of screening, all disease states are undetected except for symptom-detected cancer states. Once screening is introduced, a simulated person who has an adenoma or preclinical cancer has a chance of having it detected during screening.
Both models determine whether a simulated individual is screened and what type of screening test he or she receives, based on NHIS data. The probability that an individual has his or her first screening in any given year is a function of age, sex, race, calendar year and the scenario being run. As in real life, screening intervals vary among individuals and from one type of screening to another.
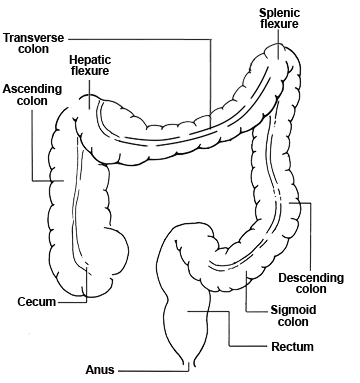 Detection depends on the size and location of the adenoma/cancer and the sensitivity and reach of the test. The three tests we modeled – FOBT, sigmoidoscopy, and colonoscopy – have distinct characteristics, reach, and risk.
Detection depends on the size and location of the adenoma/cancer and the sensitivity and reach of the test. The three tests we modeled – FOBT, sigmoidoscopy, and colonoscopy – have distinct characteristics, reach, and risk.
- FOBT has the ability to detect a lesion in any segment of the colorectal system, but has relatively poorer diagnostic sensitivity compared with the other screening modalities. A positive FOBT should be followed up with a colonoscopy. We model false-positives and the rate of compliance with follow-up procedures. In the SimCRC model, the sensitivity is applied to the most advanced lesion only. In the MISCAN model, the cumulative sensitivity due to all lesions determines the chance that the FOBT will be positive.
- Sigmoidoscopy can only detect lesions located in the sigmoid or descending colon or rectum. When a simulated person undergoes sigmoidoscopy, each lesion residing in those segments has the chance of being detected, based on the lesion-specific sensitivity. If any lesion is found, the person is then referred to colonoscopy.
- Colonoscopy has the ability to detect lesions throughout the colorectal system. Colonoscopy is also associated with a small mortality risk due to perforation of the large intestine during the procedure. We assume that all adenomas that are detected during colonoscopy are removed via polypectomy.
Parameters used for each test are given in the table below. Sensitivity is the likelihood that a test will find cancers or adenomas if they exist. Specificity is the probability of a negative result in patients free from any lesions. We assumed distinct sensitivities for small (1-5 mm), medium (6-9 mm) and large (10+ mm) adenomas as well as preclinical cancer.
| SimCRC Model | MISCAN Model | |
|---|---|---|
FOBT |
||
| Sensitivity for small, medium, large adenomas | 10,10,10% (per person) | 2,2,5% (per adenoma) |
| Cancer sensitivity | 33% | 60% |
| Specificity | 97% | 98% |
| Reach | Whole colon and rectum | Whole colon and rectum |
| Compliance with follow-up | 85% | 80% |
Sigmoidoscopy |
||
| Sensitivity for small, medium, large adenomas (within reach) | 76,87,94% | 75,85,95% |
| Cancer sensitivity (within reach) | 94% | 95% |
| Specificity | 100% | 100% |
| Reach | Distal colon and rectum | 75% reach descending colon, none beyond splenic flexure |
| Compliance with follow-up | 85% | 80% |
Colonoscopy |
||
| Sensitivity for small, medium, large adenomas (within reach) | 76,87,94% | 80,85,95% |
| Cancer sensitivity (within reach) | 94% | 95% |
| Specificity | 100% | 100% |
| Reach | Whole colon and rectum | 95% reach ascending colon, 70% reach cecum |

