Breast Cancer
The CISNET Breast Working Group (BWG) consists of six modeling groups and a coordinating center. The rapid pace of discovery about breast cancer biology has given rise to survival gains and new precision screening and treatment approaches. The BWG is funded to use collaborative computer modeling to inform translation of precision oncology evidence to clinical guidelines and policy by evaluating strategies to further decrease mortality, understand survivor quality of life, and reduce disparities. They will use six breast cancer simulation models as a virtual laboratory to inform policy and clinical decisions by evaluating the impacts on mortality, quality of life, harms, and costs of disseminating these evolving precision cancer control paradigms.
Highlights
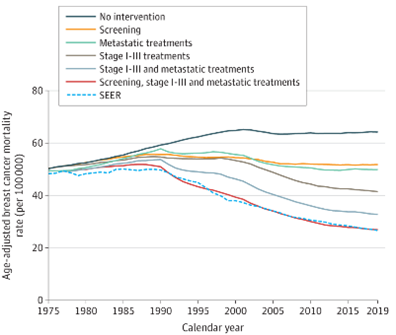 In this recent analysis published in JAMA, four CISNET models were used to estimate the contribution to the reduction in breast cancer mortality of changes in breast cancer screening and treatment since 1975.
In this recent analysis published in JAMA, four CISNET models were used to estimate the contribution to the reduction in breast cancer mortality of changes in breast cancer screening and treatment since 1975.
Investigators
Comparative Modeling: Informing Breast Cancer Control Practice and Policy
Grant Number: U01CA253911
Abstract & Aims
Abstract: The CISNET Breast Working Group (BWG) proposes innovative modeling research focused on new precision oncology paradigms that are expected to re-define breast cancer control best practices. We selected significant topics where modeling is suited to fill evidence gaps and facilitate clinical and policy translation. Unique components of our approach include modeling of absolute risk of disease accounting for multiple risk factors, addressing important comorbidities—specifically type 2 diabetes—that affect both disease risk and survival, exploring emerging biomarker-based approaches for screening, providing guidance regarding precision systemic treatments and their impact on quality of life in survivors, and investigating race disparities.
The specific aims are to:
- Evaluate the impact of novel precision screening approaches;
- Evaluate the impact of precision treatment paradigms in the adjuvant, neo-adjuvant, and metastatic setting;
- Synthesize Aims 1 and 2 to quantify the contributions of precision screening and precision treatment to US breast cancer mortality reductions; and
- Provide evidence to guide interventions to reduce race disparities by quantifying multiple risk, screening, treatment, and survival factors that impact disparities.
This scope of work would not be feasible without the availability of six distinctive BWG models: Dana Farber (D), Erasmus (E), Georgetown-Einstein (GE), MD Anderson (M), Stanford (S), Wisconsin-Harvard (W).
The aims encompass multiple RFA priority areas, and we have set aside Rapid Response funds to address remaining priority areas, support cross-cancer CISNET collaborations, and foster junior career enhancement. Each aim includes three or more model groups selected for their unique structure and includes outside collaborators and junior investigators. The models will share common inputs and provide a standard set of outcomes for benefits (e.g., distant recurrences and deaths avoided, mortality reductions, distant disease-free survival, and life years and quality-adjusted life years), harms (e.g., false positives and benign biopsies, interval cancers, advanced stage diagnoses, overdiagnosis and treatment impact on quality of life), and costs.
Continuously funded for the past 19 years, the modeling teams have published 204 research papers informing public health policy decisions and trained 13 junior investigators. For this proposal, the BWG will partner with the American Cancer Society, the American College of Radiology, the American Society of Clinical Oncology, the Breast Cancer Surveillance Consortium, and others. An experienced Coordinating Center provides the infrastructure to support the project goals including resource sharing and model accessibility. The exceptional environment provides unprecedented synergy and leveraging of resources to address new research questions and support career development that would not otherwise be possible. Overall, this research will advance modeling research and guide breast cancer control policy.
Major Analyses & Contributions
Comparative Analyses
Association of Screening and Treatment With Breast Cancer Mortality by Molecular Subtype in US Women, 2000‒2012
All six simulation modeling groups for breast cancer participated in a project to determine the associations of screening and adjuvant treatment with reductions in US breast cancer mortality rates by molecular subtype (Plevritis et al., 2018). The results suggest that for women aged 30 to 79 years, advances in treatment (such as the use of new adjuvant therapies) were associated with greater estimated reductions in overall breast cancer mortality from 2000 to 2012 compared with advances in screening. The models attributed 63% (model range: 49‒74%) of the overall breast cancer mortality reduction to treatment, with only 37% (model range: 26‒51%) of the reduction associated with screening. Of this 63% associated with treatment, 31% was associated with chemotherapy; 27% with hormone therapy; and 4% with trastuzumab. These results varied by breast cancer molecular subtype.
Contribution of Screening and Treatment on Breast Cancer Trends
Prior model-based analyses published in the New England Journal of Medicine (Berry et al., 2005) and described as a landmark study, quantified the relative effects of screening mammography and adjuvant treatment at a population level. However, these effects had not been quantified by estrogen receptor (ER) status. Breast cancer is a heterogeneous disease defined by molecular subtypes that predict treatment response and clinical outcomes and ER is the longest-established molecular marker in use for breast cancer treatment planning. To quantify the effects of screening and adjuvant treatment on US breast cancer mortality trends by ER status from 1975 to 2000, the CISNET Breast Working Group updated the landmark analysis using ER-specific model inputs (Munoz et al., 2014). All six modeling groups projected greater absolute mortality declines in ER-positive cancers than among ER-negative cancers, consistent with observed trends. For ER-positive cases, adjuvant treatment made a higher relative contribution to breast cancer mortality reduction than screening, whereas for ER-negative cases the relative contributions were similar for screening and adjuvant treatment. ER-negative cancers were less likely to be screen-detected than ER-positive cancers (35.1% vs. 51.2%), but when screen-detected, yielded a greater survival gain (five-year breast cancer survival = 35.6% vs. 30.7%).
A manuscript with analyses for 2000-2010 using ER and HER2-specific inputs and modeling newer adjuvant treatments including anthracycline-based regimens and taxanes, aromatase inhibitors for ER-positive breast cancers, and trastuzumab for HER-2 positive tumors, is currently under review.
Landmark Study
Impact of Mammography and Adjuvant Therapy on the Decline in U.S. Breast Cancer Mortality: 1975-2000.
After remaining relatively constant for many years, breast cancer mortality in the United States decreased by a dramatic 24% from 1989 to 2000. CISNET investigators initiated a joint comparative modeling effort among seven groups to determine the contributions of mammography and adjuvant therapy to this decline. While the benefits of adjuvant therapy were more settled, controversy regarding the benefits of mammography screening persisted due to uneven results and continuing criticism of the controlled trials on which the mortality benefits had been based. The dissemination and usage patterns of mammography and adjuvant therapy were coupled with seven independent modelers' syntheses of all available information on the benefits of these advances to estimate their population impact.
Dissemination of Screening and Adjuvant Therapy
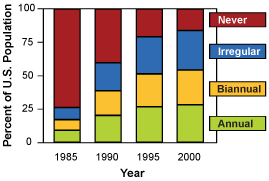
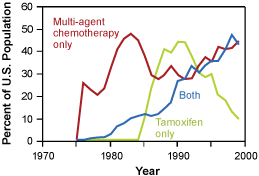
Changes in the pattern of screening mammography use among women 40 to 79 years of age, and change in the use of adjuvant therapy among women 50 to 69 years of age with node positive stage II or IIIA breast cancer. Copyright 2005 Massachusetts Medical Society. All rights reserved.
All models agree on three points:
- Both screening and treatment reduced breast cancer mortality,
- The observed reduction in the overall population could not be attributed to either one acting alone, and
- Each contributed about equally to this decline.
Typically, results based on observational data are validated using controlled trials. However, this was a case where observational data (combined in a novel way using seven different models) helped to confirm mammography benefits, when controlled trial results alone could not settle the debate.
Mortality Trends and Results of Joint Modeling Effort
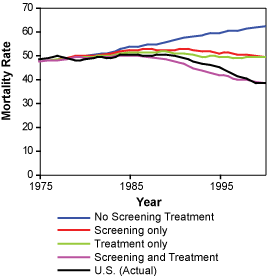
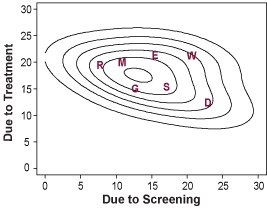
Left: Estimated and actual mortality rates from breast cancer among women 30 to 79 years of age under hypothetical assumption about the use of screening mammography and adjuvant treatment.
Right: Estimated joint distribution of the reduction in the rate of death from breast cancer among U.S. women 30 to 79 years of age that is attributable to adjuvant treatment and screening mammography. Letters represent point estimates from each of the seven CISNET models.
Copyright 2005 Massachusetts Medical Society. All rights reserved.
This analysis was first published in the The New England Journal of Medicine:
Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habberna JDF, Feuer EJ. Effect of Screening and Adjuvant Therapy on Mortality from Breast Cancer. New Eng J Med 2005 Oct 27;353(17):12-20. [Abstract]
In addition, a JNCI monograph describes the details of each of the seven models, the common inputs, and the results. The monograph provides the opportunity to examine the links between results and the model structures to give insight into the complex relationship between modeling assumptions and conclusions, exemplifying the benefits obtained from a comparative modeling approach.
Cancer Intervention and Surveillance Modeling Network (CISNET) Breast Cancer Collaborators. The Impact of Mammography and Adjuvant Therapy on U.S. Breast Cancer Mortality (1975-2000): Collective Results from the Cancer Intervention and Surveillance Modeling Network. J Natl Cancer Inst Monographs 2006;36:1-126. [Monograph
]
Other Achievements
Breast Cancer Other Achievements: Highlights
- Modeling comorbidities to determine age of screening cessation
- Race-specific modeling
- Evaluating the impact of new screening modalities
- Can the probability of overdiagnosis in early detection programs be estimated?
Modeling comorbidities to determine age of screening cessation
Two of the Breast Working Group models (E and GE) participated in an analysis with other CISNET sites to estimate the harms and benefits of cancer screening by age and comorbidity to inform decisions about screening cessation (Lansdorp-Vogelar et al., 2014). The results suggest that estimates of screening benefits and harms by comorbidity can inform discussions between providers and their older patients about personalizing decisions about when to stop cancer screening. In the analysis, screening 1000 women with average life expectancy at age 74 for breast cancer resulted in 7996 (range across models) false-positives, 0.50.8 overdiagnosed cancers, and 0.70.9 breast cancer deaths prevented. While absolute numbers of harms and benefits differed across cancer sites, the ages at which to cease screening were highly consistent across models and cancer sites when based on harm-benefit ratios comparable to screening average-health individuals at age 74. For individuals with no, mild, moderate, and severe comorbidities, screening until ages of 76, 74, 72, and 66, respectively, resulted in similar harms and benefits as for average-health individuals.
Race-specific modeling
We used three Breast Working Group models (E, GE, W) to evaluate race disparities in disease natural history (Batina et al., 2013), mortality (Chang et al., 2012; van Ravesteyn et al., 2011) and how federal policies might affect screening outcomes for different minority groups, including Latinos (van Ravesteyn et al., 2015). In the first of these studies, two simulation models (E and GE) were adapted to model Black and White women (van Ravesteyn et al., 2011). The models used common national race, and age-specific data for incidence, screening and treatment dissemination, stage distributions, survival, and competing mortality from 1975 to 2010 to investigate how much of the mortality disparity can be attributed to racial differences in natural history, uptake of mammography screening, and use of adjuvant therapy. The results suggested that Black women appear to have benefited less from cancer control advances than White women, with a greater race-related gap in the use of adjuvant therapy than screening. However, a greater portion of the disparity in mortality appears to be due to differences in natural history and undetermined factors.
Evaluating the impact of new screening modalities
The CISNET Breast Working Group has studied the impact of adopting new imaging technology. We modeled the observed transitions from plain film to digital mammography and found that digital yielded only modest increases in health while substantially increasing costs (Tosteson et al., 2008; Stout et al., 2014). Models E, GE, and W examined the impact of supplemental screening ultrasound for women with dense breasts (Sprague et al., 2015). The models consistently showed this strategy was most efficient when targeted to the 10-14% of the population with extremely dense breasts, but even this strategy was not cost-effective by most standards. We concluded that the current breast density notification legislation will substantially increase costs while producing relatively small benefits in breast cancer deaths averted and QALYs gained (Sprague et al., 2015). Model W also conducted a preliminary cost-effectiveness of biennial screening for women aged 50-74 with dense breasts using digital mammography plus tomosynthesis (Lee et al., 2015). Assuming improvements in screening sensitivity and specificity for tomosynthesis at current additional out-of-pocket costs, the results suggest that combined screening would cost $53,893 per added QALY gained vs. digital mammography alone. Models E and GE also worked with the CDC to assess the impact of replacing film with digital mammography on health effects (deaths averted, life-years gained); costs (for screening and diagnostics); and number of women reached in the CDC’s National Breast and Cervical Cancer Early Detection Program (van Ravesteyn et al., 2015).
Can the probability of overdiagnosis in early detection programs be estimated?
The probability of overdiagnosis, defined as the situation where a screening exam detects a disease that would have otherwise been undetected in a person's lifetime, is an ongoing concern with early detection of breast cancer. The CISNET Breast Working Group has conducted work to better estimate the amount of overdiagnosis of DCIS in the US population. Model E has incorporated the effect of DCIS grade on natural history into the Dutch MISCAN model using recent data from the Netherlands. Model W has conducted extensive studies of DCIS risk factors (Sprague et al., 2009; Berkman et al., 2014; McLaughlin et al., 2014; Sprague et al., 2013; Sprague et al., 2010; Nichols et al., 2007; Trentham-Dietz et al., 2007; Sprague et al., 2007; Trentham-Dietz et al., 2000) and Model D is in the process of conducting a synthesis of DCIS data.
In closely related work, we have developed methods for estimating overdiagnosis. In an analysis estimating overdiagnosis among older women, all participating models found that screening women after age 74 years resulted in a less favorable balance of benefits and harms than screening women between the ages of 50 and 74 years because of the increasing amount of overdiagnosis at older ages (van Ravesteyn et al., 2015). Model E has examined overdiagnosis in the Dutch screening program (de Koning et al., 2006; de Gelder et al., 2011; de Gelder et al., 2011). Model D has developed a new method to quantify overdiagnosis of invasive breast cancer from mammography screening data in Norway and Model W has analyzed the impact of DCIS treatment patterns on breast cancer mortality (Sprague et al., 2013).
Lastly, we are currently working to refine the DCIS portions of the simulation models.
Model Profiles & Registry
Model profiles are standardized documents that facilitate the comparison of models and their results. The Joint Profile provided includes profiles for all breast cancer models. Individual profiles for each model are also provided and may be more current than the joint profile document.
The following model profiles have been developed by CISNET members for breast cancer:
Model Comparison Grid (PDF, 112 KB)
Joint Profiles - all profiles combined (PDF, 2.3 MB)
Individual Models
- UWBCS (Wisconsin) (PDF, 592 KB)
- BCOS (Stanford) (PDF, 510 KB)
- CISNET-DFCI (Dana-Farber) (PDF, 545 KB)
- MISCAN-Fadia (Erasmus) (PDF, 390 KB)
- Spectrum/G-E (Georgetown/Einstein) (PDF, 232 KB)
- Bayesian Simulation Model (MDACC) (PDF, 363 KB)
Historical Versions
Historical versions of model profiles and grids will be listed here.
For additional high-level information about the breast models visit the breast overview page on the CISNET Model Registry.