Prostate Cancer
The CISNET Prostate Working Group (PWG) has focused on modeling prostate cancer progression, screening, and treatment in the U.S. population, assuming biologically plausible assumptions validated against various data sources. The most effective ways to personalize the prevention and treatment of prostate cancer using data from an individual patient are unknown. The PWG applies a unique inter-disciplinary approach that combines available data with modern statistical techniques to understand long-term effects of prostate cancer interventions. In the current round, the PWG will evaluate personalizing patient care, including screening high-risk men more frequently, using biomarkers and imaging tests to select patients for biopsy, using patient and cancer features to determine when and which kinds of treatment to offer, and practical approaches for reducing racial disparities. This work will advance the evidence necessary to make informed decisions about individualized screening and treatment for this most common cancer in men.
Investigators
Modeling to Improve Prostate Cancer Outcomes Across Diverse Populations
Grant Number: U01CA253915
Abstract & Aims
Abstract: Prostate cancer is the most common solid tumor in men and the second most common cause of cancer death in the United States. The Cancer Intervention and Surveillance Modeling Network (CISNET) Prostate Working Group (PWG) was formed in the year 2000 to address a wide range of questions about effective prostate cancer control. The PWG studied the rapid increase in prostate cancer diagnoses after PSA screening started in the late 1980s to estimate lead time and overdiagnosis associated with the test. The PWG studied the decline in prostate cancer mortality that began in the early 1990s to quantify the plausible contributions of PSA screening and changes in primary treatments. The PWG also studied how to interpret trends in racial disparities in incidence and survival, how to manage men with low-risk disease on active surveillance, and how to reconcile apparently discordant randomized trials of PSA screening and radical prostatectomy. In recent years, technologies surrounding prostate cancer screening and treatment have evolved rapidly, and opportunities to improve patient care using personalized data abound. Genetic testing can identify men at increased risk for developing aggressive disease, new biomarkers and imaging tools can help men avoid unnecessary biopsies, and new hormonal treatments can lengthen survival for men with advanced disease. The objective of this application is to extend PWG models to evaluate optimal ways to utilize personalized data to improve patient care while limiting harms and costs. We will determine whether we can improve early detection using novel stratification approaches and whether we can safely limit overtreatment and other harms by tailored choices of primary and secondary therapies. These approaches will be applied in the United States and in international cancer control settings with different resources and priorities.
Our specific aims are as follows:
- Precision early detection, including risk-stratified screening and biopsy using genetic tests, novel biomarkers, and imaging technology.
- Precision active surveillance, including adaptive biopsy intervals and imaging technology.
- Precision treatment, including type and timing of initial and salvage therapies.
- Targeting screening, biopsy, and treatment policies to reduce racial disparities.
- Prioritizing screening and treatment interventions in international settings.
These aims are highly responsive to the funding opportunity announcement, addressing 7 of the 9 targeted priority areas to varying degrees. Our cumulative expertise in prostate modeling, our existing models, and our close ties with clinical experts who provide access to large, high-quality datasets for model validation and calibration put us in a strong position to answer critical and impactful questions about how best to control this most common cancer in men.
Major Analyses & Contributions
Comparative Analyses
Reconciling the effects of screening in the ERSPC and PLCO trials
Some 20 years after PSA screening began in the U.S., two large randomized trials published apparently conflicting results: The European Randomized Study of Screening for Prostate Cancer (ERSPC) found that screening reduced prostate cancer mortality, but the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) found no reduction. Interpreting the results is complicated by differences in their implementation. Compared to the ERSPC, the PLCO involved a shorter interval between screens, a higher PSA threshold for biopsy referral, less frequent biopsy, and more frequent screening in the control group. In collaboration with investigators from both trials, we investigated differences between trials using two approaches. First, we estimated aggregate effects of screening on incidence in each arm of each trial in terms of a mean lead time. Then we analyzed whether the association between the risk of prostate cancer death and the mean lead time differed between trials. Finding no evidence of a different association, we concluded that the trials provide compatible evidence that screening reduces prostate cancer mortality (Tsodikov et al., 2017). Second, we applied screening efficacy estimated using ERSPC data to simulated trials with systematically varied settings for known differences in trial implementation, including screening intervals, PSA thresholds, biopsy receipt, and control arm contamination. We concluded that trial results are sensitive to implementation, and differences in biopsy frequency and control arm contamination were major contributors to the apparent differences in published results for the two trials (de Koning et al., 2018).
Modeling prostate cancer natural history in black men
National guidelines for prostate cancer screening do not provide explicit recommendations for black men, whose risk of dying of the disease is approximately twice that in the general population. Since empirical studies comparing screening strategies in a black population are not feasible, we used modeling to investigate whether black men should be screened differently than the general population. We developed race-specific versions of our natural models and calibrated them to population incidence trends among black men and the general population. Our results show that black men have considerably higher risk of disease onset and progression to metastasis. These results support initiating screening earlier in black men and potentially screening them more frequently (Tsodikov et al., 2017).
Projecting expected effects of discontinued vs age-restricted screening
Concerns about overdiagnosis and overtreatment of prostate cancer associated with PSA screening and limited absolute benefit reported from screening trials motivated major revisions to U.S. guidelines. Most notably the U.S. Preventive Services Task Force recommended against routine PSA screening in 2012, while other guidelines panels (e.g., the American Cancer Society, the American Urological Association, and American College of Physicians) recommended shared decision-making for healthy men with at least 10-year life expectancy, typically up to age 70 or 75 years. These inconsistent guidelines have created uncertainty among health care providers with millions of men who are candidates for screening. Two models projected the expected impacts of completely discontinued PSA screening vs a continuation of historical PSA screening restricted to men under 70 years of age. The models projected that completely discontinued PSA screening may result in many avoidable prostate cancer deaths. In contrast, age-restricted PSA screening would eliminate 64%-66% of overdiagnoses relative to a continuation of historical PSA screening and prevent 61%-64% of avoidable prostate cancer deaths relative to completely discontinued PSA screening. The results support finding ways to continue screening that reduce harms while preserving as much of the benefit of screening as possible (Gulati et al., 2014).
Healthy men and their primary care physicians must choose between conflicting clinical guidelines
Some U.S. clinical guidelines recommend against routine PSA screening while others recommend shared decision-making for healthy men, typically up to age 70 years.
Explaining observed declines in prostate cancer mortality
Prostate cancer death rates have declined by nearly one-half since the early 1990s. Many assume that PSA screening, which became popular in the early 1990s, is responsible for this drop in prostate cancer deaths. However, treatment for prostate cancer has also been changing. In the 1980s, radical prostatectomy increased in prominence, while, during the 1990s, hormonal therapies, previously reserved for advanced disease, were added to treatment regimens for localized tumors. Three models were used to quantify the fraction of the mortality decline attributable to changes in initial treatment. The models projected that changes in treatment explained 22% to 33% of the mortality decline by 2005. By isolating and quantifying the effects on mortality of treatment changes, we were able to more clearly quantify the likely role of PSA screening in the population setting (Etzioni et al., 2012).
Examining effects of control arm contamination in the PLCO cancer screening trial
The prostate section of the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial found a non-significant increase in prostate cancer mortality in the intervention arm compared to the control arm. However, 45% of participants reported receiving at least one prior PSA test before entering the trial, control arm participants continued to receive PSA tests at a higher frequency than the general population, and prostate cancer mortality was lower than expected in both arms. Three models were applied to study the effects of pre-trial and control arm screening on the published mortality rate ratio. The models found that under a clinically significant mortality reduction associated with PSA screening similar to that observed in the ERSPC trial, pre-trial and control arm screening substantially reduced the power of the PLCO to detect a mortality difference between arms and lead to a nontrivial chance of finding excess mortality in the intervention arm (Gulati et al., 2012).
Characterizing prostate cancer natural history
Informed decisions about PSA screening for prostate cancer and about treatment following detection by screening require information about disease natural history, including the chances that the cancer has been overdiagnosed or that the cancer would lead to death if untreated. We leveraged three models to summarize key events in the development and progression of prostate cancer and to project risks of clinical progression and disease-specific deaths for PSA-detected cancers in the absence of treatment. The models projected that the lifetime risk of developing a preclinical prostate tumor is 20%-33% (range across models), that 38%-50% of these tumors would be diagnosed in the absence of screening and that 12%-25% would lead to death in the absence of treatment. Risks of overdiagnosis, metastasis, and prostate cancer death depended on age, Gleason grade, and PSA at diagnosis (Gulati et al., 2011).
Prostate cancer and non-prostate cancer survival after PSA detection in the absence of treatment
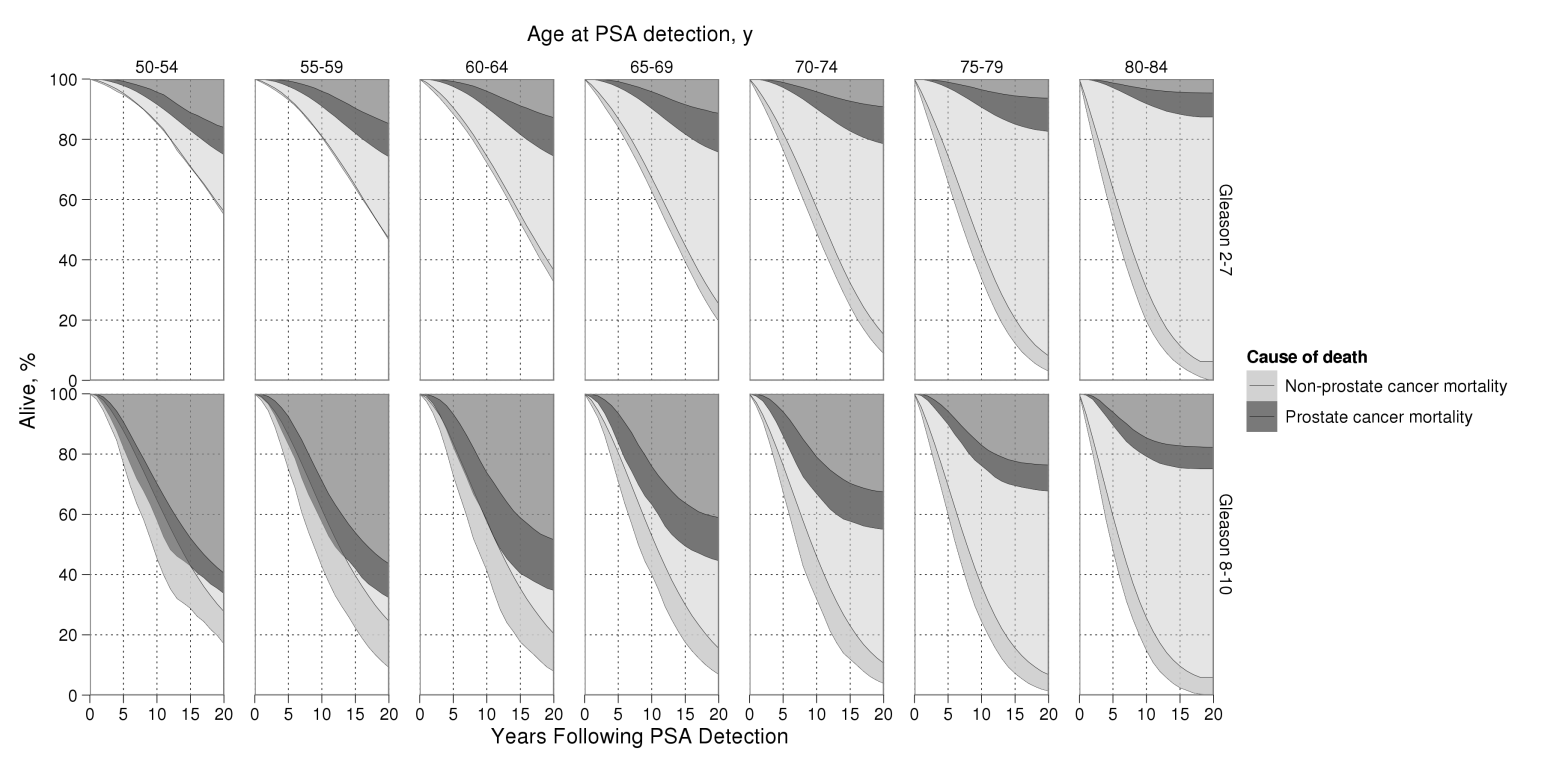
Prostate cancer and non-prostate cancer mortality after diagnosis by PSA screening in the absence of treatment by age and grade at diagnosis. For either cause of death, lighter areas reflect agreement by all 3 models and darker areas reflect between-model uncertainty.
Reprinted from Cancer Epidemiology Biomarkers and Prevention, 2011, Volume 20, Issue 5, pp. 740-50, Roman Gulati, Elisabeth M. Wever, Alex Tsodikov et al., What If I Dont Treat My PSA-Detected Prostate Cancer? Answers from Three Natural History Models, with permission from the American Association for Cancer Research.
Reconciling differing estimates of lead time and overdiagnosis due to PSA screening
Lead time and overdiagnosis are unobservable quantities that are key to evaluating the trade-offs between the potential benefits and harms of PSA screening. Previous studies, including two studies by CISNET prostate investigators (Tsodikov et al., 2006; Etzioni et al., 2002), published mean lead time estimates that ranged from 3 to 7 years and overdiagnosis estimates that ranged from 25% to 84% of all screen-detected cases. These prior estimates were highly disparate because they were developed under differing systems of practice of PSA use (e.g., PSA cutoffs and biopsy practices), different populations, as well as different assumptions and definitions of lead time and overdiagnosis. CISNET investigators recognized this opportunity to bring order to this disparate literature by developing estimates using U.S. practice patterns and a consistent set of definitions. By standardizing in this manner, three CISNET modeling groups were able to reduce the range of mean lead times to 5-7 years and overdiagnosis frequencies to 23%42% (Draisma et al., 2009). The two major randomized trials of prostate cancer screening (PLCO and ERSPC) use considerably different screening protocols, including PSA thresholds for biopsy and biopsy practices, and produced apparently conflicting results about the benefit of PSA screening. This CISNET prostate working group is working with investigators from both trials to reconcile differences in their circumstances of implementation and to assist in translating estimates of both benefit and harm to inform public health guidelines for the use of PSA screening.
The Iceberg Effect
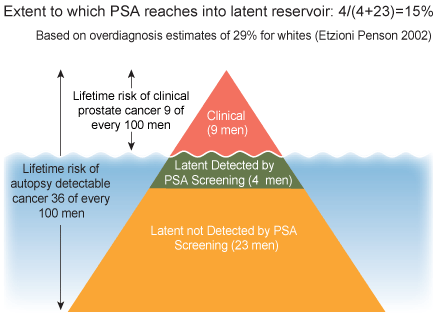
Extent to which PSA screening reaches the reservoir of latent prostate cancer based on overdiagnosis estimates of 29% and lifetime estimates of latent disease of 36% (Etzioni et al., 1998).
Quantifying the role of prostate-specific antigen (PSA) screening in U.S. prostate cancer mortality
The value of PSA screening remains uncertain. Even before randomized clinical trials on the potential benefits of PSA screening on prostate cancer mortality began, the test was rapidly adopted in several countries including the United States. However, while there is a general consensus that PSA screening explains much of the decline in the incidence of distant prostate cancers, there is still considerable debate about its role in the observed mortality decline. Two models were used to determine the plausible contribution of PSA screening in the decline of U.S. mortality. The researchers used common estimates of PSA screening rates and assumed that, by shifting the disease from a distant to a localized/regionalized clinical stage, screening does result in a corresponding improvement in disease-specific survival and mortality. The researchers concluded that PSA screening may account for much but not all of the observed reduction in prostate cancer mortality. Other factors, such as changing treatment practices, also may have played a role in improving prostate cancer outcomes (Etzioni, et al., 2008).
Other Achievements
- What are the real effects of PSA screening on racial disparities in prostate cancer survival?
- Are risks of biopsy upgrading consistent across active surveillance studies?
- Can conservative treatment improve the cost-effectiveness of screening?
- Under what conditions are empirical estimates of overdiagnosis unbiased?
- How do the methods used to estimate overdiagnosis affect the results?
- How does overdiagnosis depend on patient and tumor characteristics?
- Can we quantify the risk of overdetection of PSA recurrence after radical prostatectomy?
- How do age and comorbidity impact harms and benefits of PSA screening?
- Can PSA screening for prostate cancer be cost-effective?
- Can we find "smarter" PSA screening strategies that reduce harms while preserving benefit?
- How much higher is prostate cancer mortality after active surveillance vs immediate surgery?
- Can ecologic analysis be used to determine the likely efficacy of PSA screening?
- Can racial disparities in PSA screening explain racial differences in prostate cancer mortality declines?
- Is tumor dedifferentiation actually being prevented by early detection and consequent treatment?
- Do prostate cancer tumor characteristics affect PSA growth?
- Are there racial disparities in prostate cancer care?
What are the real effects of PSA screening on racial disparities in prostate cancer survival?
Racial disparities in prostate cancer survival narrowed during the PSA era, suggesting that screening may induce more equitable outcomes. However, artifacts of screening—lead time and overdiagnosis can inflate survival without reflecting real benefit. We developed a simulation model to disentangle these artifacts from real survival improvements by age and race. We found that lead time and overdiagnosis explained nearly all of the apparent survival improvements at older ages. Depending on the screening benefit, real survival improvements in the PSA era ranged from 27%–40% among black men and 26%–38% among all races. We concluded that real improvements in survival disparities in the PSA era are smaller than those observed and reflect similar reductions in the risk of prostate cancer death among blacks and all races (Kaur et al., 2018).
Are risks of biopsy upgrading consistent across active surveillance studies?
Active surveillance is an increasingly accepted approach for managing low-risk prostate cancer, yet there is no consensus about implementation. This lack of consensus is due in part to uncertainty about risks for disease progression, which have not been systematically compared or integrated across studies with different surveillance protocols and different frequencies of dropout to active treatment. We developed and fit a joint model of PSA levels and risks for biopsy upgrading (from Gleason score ≤6 to ≥7) to patient records from Johns Hopkins University (JHU); Canary Prostate Active Surveillance Study (PASS); University of California, San Francisco (UCSF); and University of Toronto (UT) active surveillance studies. After accounting for differences in surveillance intervals and competing treatments, estimated risks for biopsy upgrading were similar in the PASS and UT studies but higher in UCSF and lower in JHU studies. However, despite heterogeneity in risks of upgrading, evidence across studies is consistent with only a small delay in upgrading associated with biennial compared with annual biopsies (Inoue et al., 2018).
Can conservative treatment improve the cost-effectiveness of screening?
Some researchers have suggested that personalized screening combined with conservative management for low-risk cancers could improve the net value of PSA screening. We evaluated 18 screening strategies combined with (1) contemporary treatment practices based on age and cancer stage and grade observed in the Surveillance, Epidemiology, and End Results Program in 2010 or (2) selective treatment practices whereby cancers with Gleason score <7 and clinical stage ≤T2a are treated only after clinical progression. Combined with contemporary treatment, only highly conservative screening strategies were cost-effective relative to no screening. Combined with selective treatment, many more strategies became cost-effective. For PSA screening to be cost-effective, it needs to be used conservatively and ideally in combination with a conservative management approach for low-risk disease (Roth et al., 2016).
Under what conditions are empirical estimates of overdiagnosis unbiased?
Overdiagnosis is often estimated by calculating the excess incidence in a screened group relative to an unscreened group. Yet conditions for unbiased estimation are poorly understood. We developed a conceptual framework to project the effects of screening on the incidence of so-called relevant cancers—cancers that would present clinically without screening—in common trial and population settings. Screening advances the date of diagnosis for a fraction of preclinical relevant cancers; which diagnoses are advanced and by how much depends on the preclinical detectable period, test sensitivity, and screening patterns. Using the model, we compared excess incidence with true overdiagnosis. In trials with no control arm screening, unbiased estimates are available using cumulative incidence if the screen arm stops screening and using annual incidence if the screen arm continues screening. In both designs, unbiased estimation requires waiting until screening stabilizes plus the maximum preclinical period. In continued-screen trials and population settings, excess cumulative incidence is persistently biased. In general, no trial or population setting automatically permits unbiased estimation of overdiagnosis; sufficient follow-up and appropriate analysis remain crucial (Gulati et al., 2016).
How do the methods used to estimate overdiagnosis affect the results?
Frequencies of overdiagnosis of breast and prostate cancers, cancers that would not have presented in the absence of screening, have been estimated in numerous studies. This study explores study features and methods that influence published estimates. It finds that (1) the definition of the overdiagnosis, (2) the measurement of overdiagnosis, (3) the study design and context, and (4) the estimation approach are the most influential features. The study summarizes known issues with “excess-incidence” and “lead-time” approaches for estimating overdiagnosis, and it concludes with a suggested list of questions that readers of overdiagnosis studies should evaluate to better understand the likely validity of reported estimates (Etzioni et al., 2013). A separate study presented a targeted commentary on the limitations of these two estimation approaches (Etzioni et al., 2015).
How does overdiagnosis depend on patient and tumor characteristics?
Although overdiagnosis is not directly observable, it arises as the result of two competing risks in a screen-detected individual, namely the risk of disease progression to a clinical or symptomatic state and the risk of other-cause death. Consequently, the likelihood of overdiagnosis may be expected to vary with disease characteristics related to progression and patient characteristics, like age and comorbidity, related to the risk of non-cancer mortality. In this study, we used one model to project the fraction overdiagnosed among screen-detected cases given age, disease grade, and PSA level. We found that depending on these characteristics, the chance of overdiagnosis varied from 3% to 88% (Gulati et al, 2014).
Can we quantify the risk of overdetection of recurrence after radical prostatectomy?
PSA recurrence after radical prostatectomy can occur many years before progression to overt metastasis. If a patient dies of an unrelated cause before his cancer would have progressed, we say that his recurrence was overdiagnosed and any additional treatment could only have caused harm. To quantify the frequency of overdiagnosis of recurrence after prostatectomy, and to examine how it depends on patient age, PSA at diagnosis, and tumor stage and grade at diagnosis, this study compared time to metastasis in a well-studied cohort of patients who did not receive salvage treatment at recurrence and time to non-cancer death from U.S. life tables adjusted for this patient population. The comparison suggested that a non-trivial fraction of men with PSA recurrence after prostatectomy were overdiagnosed, reaching as high as 30% for men over 70 years of age at diagnosis with PSA failure within 5-10 years of diagnosis (Xia et al., 2014).
How do age and comorbidity impact harms and benefits of screening?
Harms and benefits of screening are known to depend on age and comorbid conditions, but reliable estimates of when to stop screening for prostate or other cancers had not been carefully studied. In a collaboration involving 7 models and 3 cancer sites, we examined false positive tests and overdiagnoses (harms) and cancer deaths prevented and life-years gained (benefits) under population screening programs that terminated at ages between 66 and 90 for individuals with 1 of 4 levels of comorbid conditions. The models projected that individuals with few comorbid conditions can continue screening later and individuals with more comorbid conditions can stop screening earlier to match the harm-benefit tradeoffs estimated for average-health individuals (Lansdorp-Vogelaar et al., 2014).
Can PSA screening for prostate cancer be cost-effective?
Although the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial showed a statistically significant 29% prostate cancer mortality reduction, overdiagnosis due to screening can impact quality of life. Alternative screening strategies for the population may exist that optimize the effects on mortality reduction, quality of life, overdiagnosis, and costs. Based on data from the ERSPC trial, we used one model to predict the cost-effectiveness of 68 screening strategies starting at age 55 years. The screening strategies varied by age to stop screening and screening interval (1 to 14 years or once in a lifetime screens) and therefore the number of tests. The results indicated that prostate cancer screening can be cost-effective when it is limited to two or three screens between ages 55 to 59 years. Screening above age 63 years is less cost-effective because of loss of QALYs because of overdiagnosis in this setting (Heijnsdijk et al., 2014).
Can we find “smarter” PSA screening strategies that reduce harms while preserving benefit?
The U.S. Preventive Services Task Force recommended against routine PSA screening in 2012 based on harms and benefits of then-current PSA-based screening practices but called for additional research into alternative use of existing screening tools and practices that improve harm-benefit tradeoffs. One model was used to project plausible harms and benefits under 35 alternative PSA screening strategies that differed by ages to start and stop screening, screening frequency, and criteria for biopsy referral. The results indicated that PSA screening strategies that use higher thresholds for biopsy referral for older men and that screen men with low PSA levels less frequently can reduce harms while preserving lives saved (Gulati et al., 2013).
How much higher is prostate cancer mortality after active surveillance vs immediate surgery?
Active surveillance has become increasingly accepted as a viable alternative to radical treatment for low-risk prostate cancers and a key component of a concerted effort to reduce overtreatment. However, the risk of prostate cancer death following active surveillance is unknown. This study developed a simulation model to combine data on patterns of progression while on active surveillance and implications for prostate cancer death after delayed radical treatment. The model projected that 3.4% of men on active surveillance would die of prostate cancer compared with 2.0% of men who receive immediate radical treatment. Yet the former would enjoy, on average, 6.4 years without the adverse effects of treatment (Xia et al., 2012).
Can ecologic analysis be used to determine the likely efficacy of PSA screening?
In the absence of conclusive findings from ongoing randomized trials of PSA screening, ecological studies comparing rates of prostate-cancer death between regions or countries with different screening intensities may play a role in the debate about the benefits of screening. This study compared PSA screening and prostate cancer mortality rates in nine SEER areas in the United States and found moderate association between the extent of PSA use and prostate cancer mortality declines. A computer model was used to determine whether divergence of mortality declines would be expected under an assumption of a clinically significant survival benefit due to screening. The model projected that in the presence of modest differences in screening frequencies, the mortality differences are likely to be small and might be swamped by other effects such as treatment changes. The authors concluded that ecologic studies of PSA screening, particularly those with negative results, should be interpreted with extreme caution (Shaw et al., 2004; Etzioni, Feuer 2008).
Can racial disparities in PSA screening explain racial differences in prostate cancer mortality declines?
By combining Medicare claims and National Health Interview Survey data, patterns on PSA screening were reconstructed for African-American and white men in the United States. Results indicate that uptake of screening among young African-American men (under age 65) was comparable to that among young white men and that overall screening dissemination among African-Americans only lagged slightly behind that among whites (Mariotto et al., 2007). These similarities indicate that racial disparity in PSA testing is probably not a major factor behind racial differences in prostate cancer mortality declines.
Is tumor dedifferentiation actually being prevented by early detection and consequent treatment?
Tumor differentiation as measured by the Gleason score is highly predictive of the course of prostatic cancer after diagnosis. Data from the European Randomized Study of Screening for Prostate Cancer (ERSPC) was fit to the Erasmus MC, University Medical Center Rotterdam prostate cancer model (MISCAN) under two different model assumptions: Model I, where tumors dedifferentiate before becoming screen-detectable, and Model II, where dedifferentiation occurs during the screen-detectable pre-clinical phase. Model II fit the ERSPC data significantly better than Model I, where tumors dedifferentiate before becoming screen-detectable, and Model II, where dedifferentiation occurs during the screen-detectable pre-clinical phase. Model II fit the ERSPC data significantly better than Model I, providing epidemiological evidence that tumors dedifferentiate during the screen-detectable phase and, consequently, screening with PSA and early treatment can possibly prevent progression to poorer Gleason scores (Draisma et al., 2006).
Do prostate cancer tumor characteristics affect PSA growth?
The Fred Hutchinson Cancer Research Center group combined three retrospective studies of PSA growth prior to prostate diagnosis: the Nutritional Prevention of Cancer Trials, the Beta-Carotene and Retinol Efficacy Trial, and the Baltimore Longitudinal Study of Aging. This showed accelerated PSA growth among cases later diagnosed with late-stage or high-grade disease than among those later diagnosed with early-stage or low-grade disease. The findings have important implications for screening strategies because they suggest that the window of opportunity to identify more aggressive cancers or those destined to spread may be shorter than that for more indolent cancers (Inoue et al., 2004).
Are there racial disparities in prostate cancer care?
Using linked SEERMedicare data, estimated trends of prostate cancer treatments have confirmed the findings of other studies showing rapid growth in the uptake of adjuvant and neo-adjuvant hormonal therapy in the 1990s (Zeliadt et al., 2004). However, the same study also showed that African-American men were significantly less likely than white men to receive aggressive therapy for their tumors during the 1990s. In a separate study (Zeliadt et al., 2003), a noticeable difference between African-American and whites in the frequency of post-diagnosis surveillance was found. Taken together, these results are consistent with the hypothesis that treatment disparities play a role in the poorer outcomes experienced by African-American prostate cancer patients.
Model Profiles & Registry
Model profiles are standardized documents that facilitate the comparison of models and their results. The Joint Profile provided includes profiles for all prostate cancer models. Individual profiles for each model are also provided and may be more current than the joint profile document.
The following model profiles have been developed by CISNET members for prostate cancer:
Model Comparison Grid (PDF, 109 KB)
Joint Profiles - all profiles combined (PDF, 11.3 MB)
Individual Models
- MISCAN-PRO (Erasmus) (PDF, 1.0 MB)
- PSAPC (FHCC) (PDF, 9.3 MB)
- SCANS (Michigan) (PDF, 1.9 MB)
- MSM-CanProg (UK) (PDF, 1.4 MB)
Historical Versions
Individual Models
- MISCAN-PRO (Erasmus) (PDF, 312 KB)
- PSAPC (FHCC) (PDF, 1.4 MB)
For additional high-level information about the prostate models visit the prostate overview page on the CISNET Model Registry.